The Gastro-Intestinal Simulator (GIS) is an in vitro multi-compartmental dissolution apparatus used to measure the dissolution rate of pharmaceutical solid dosage forms. It represents the next level in vivo predictive dissolution methodology.
About the GIS
The Gastrointestinal Simulator (GIS) is an in vitro multi-compartmental dissolution apparatus used to measure the dissolution rate of pharmaceutical solid dosage forms. It incorporates in vivo predictive dissolution methodology based on accumulated knowledge and continuing studies.
The GIS system consists of three separate chambers:
- A gastric chamber, to represent the stomach
- A duodenal chamber, to represent the proximal small intestine
- and a jejunal chamber, to represent the proximal to mid-small intestine
The GIS also contains two secretory chambers to hold secretion fluids that are pumped into the gastric and duodenal chambers during the experiment
The GIS provides a better representation of the physiological environment than conventional, single compartment dissolution devices, which typically contain an unrealistically large volume of a single type of fluid.
“Our goal is to develop an apparatus that a formulation scientist can use before going into human studies to determine what the best product to use in humans is.”
Fluid properties that can be designed to mimic the desired gastrointestinal segment include:
- pH
- buffer species and concentration
- bile and lipid concentration
System Set-up
The GIS is typically set-up as follows:
- The gastric chamber is filled with a mixture of 50 ml of a simulated gastric fluid and 250 ml of water that a person ingests with a dosage form
- The gastric secretion chamber is filled with ph 2 hydrochloric acid
- The duodenal chamber is filled with 50 ml of a simulated intestinal fluid, typically at a ph between 6 and 7
- The duodenal secretion chamber is likewise filled with the simulated intestinal fluid
- The jejunal chamber is left empty at the beginning of the experiment
- The temperature of the system is controlled at 37 degree Celsius
- Before the experiment commences each peristaltic pump is calibrated and the desired flow rates are set using a graphical user interface
Analysis
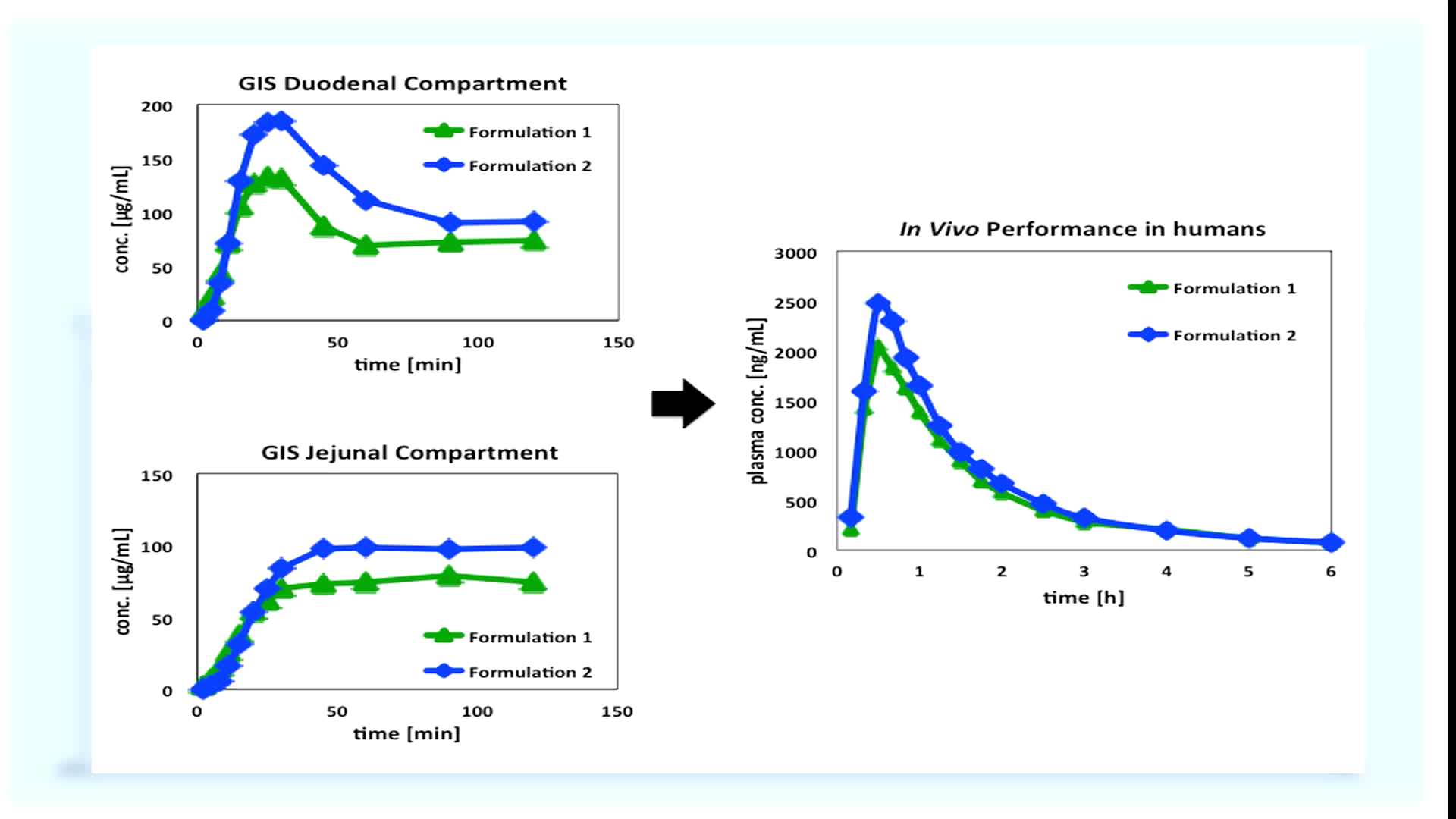
The dissolved drug concentration profile in each chamber can be used to predict the in vivo performance of the drug product. Higher dissolved concentrations in the duodenal and jejunal chambers can be indicative of enhanced in vivo performance. The apparatus we have just demonstrated is a prototype the University of Michigan has been improving on over the last three years. We are currently developing a more enhanced and reliable GIS. The next generation system will provide formulation scientists a robust platform for comparing and selecting oral drug product formulations.
Notes
The GIS has a different objective and goal than the quality control apparatus which has many other measurements associated with quality control of a pharmaceutical product.